The FOXO4-p53 lead program against “scarred” senescent and cancer cells
Cleara’s general aim is to better understand what different types of senescence exist, how these contribute to pathology and to use its platform to selectively develop compounds for their individual elimination (as opposed to a one-size-fits-all approach of elimination). We are especially focused on a particular type of senescence, which we now call “scarred” senescence.
Scarred cells are enriched in a phosphorylated form of p53. Starting from the natural FOXO4-p53 interaction, Cleara developed two lead candidates, CL04177 and CL04183 that specifically show enhanced binding to this type of phosphorylated p53.
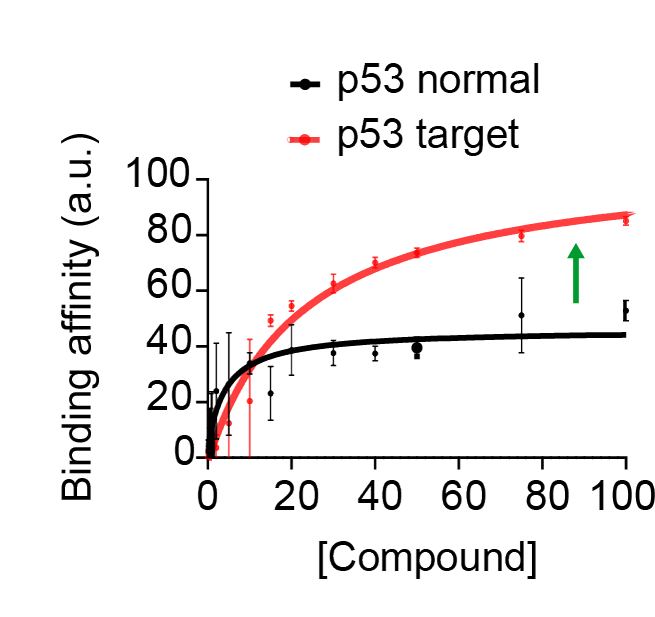
CL04183 and CL04177 show enhanced binding to target p53 vs. p53 found in normal cells, an important finding with respect to safety.

Importantly, these compounds selectively eliminate cells that are high in a proxy biomarker for scarring, i.e. PML. Thus, Cleara’s peptides cells and PML is a valuable proxy marker to indicate efficacy.
Application of CL04177/183 against “scarred” cancer
We identified that a substantial number of human tumors express PML. This prompted us to determine whether CL04177/183 could be efficacious against those types of cancer.
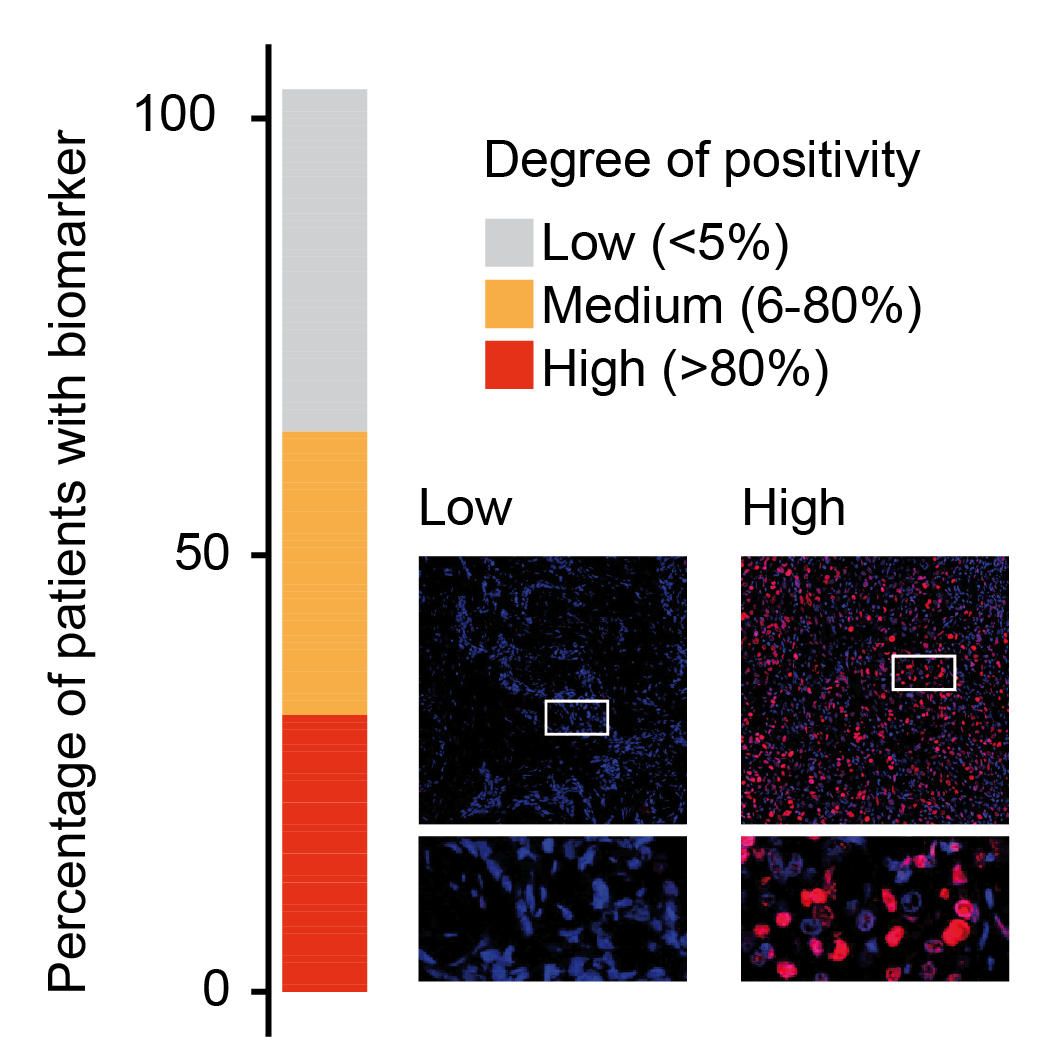
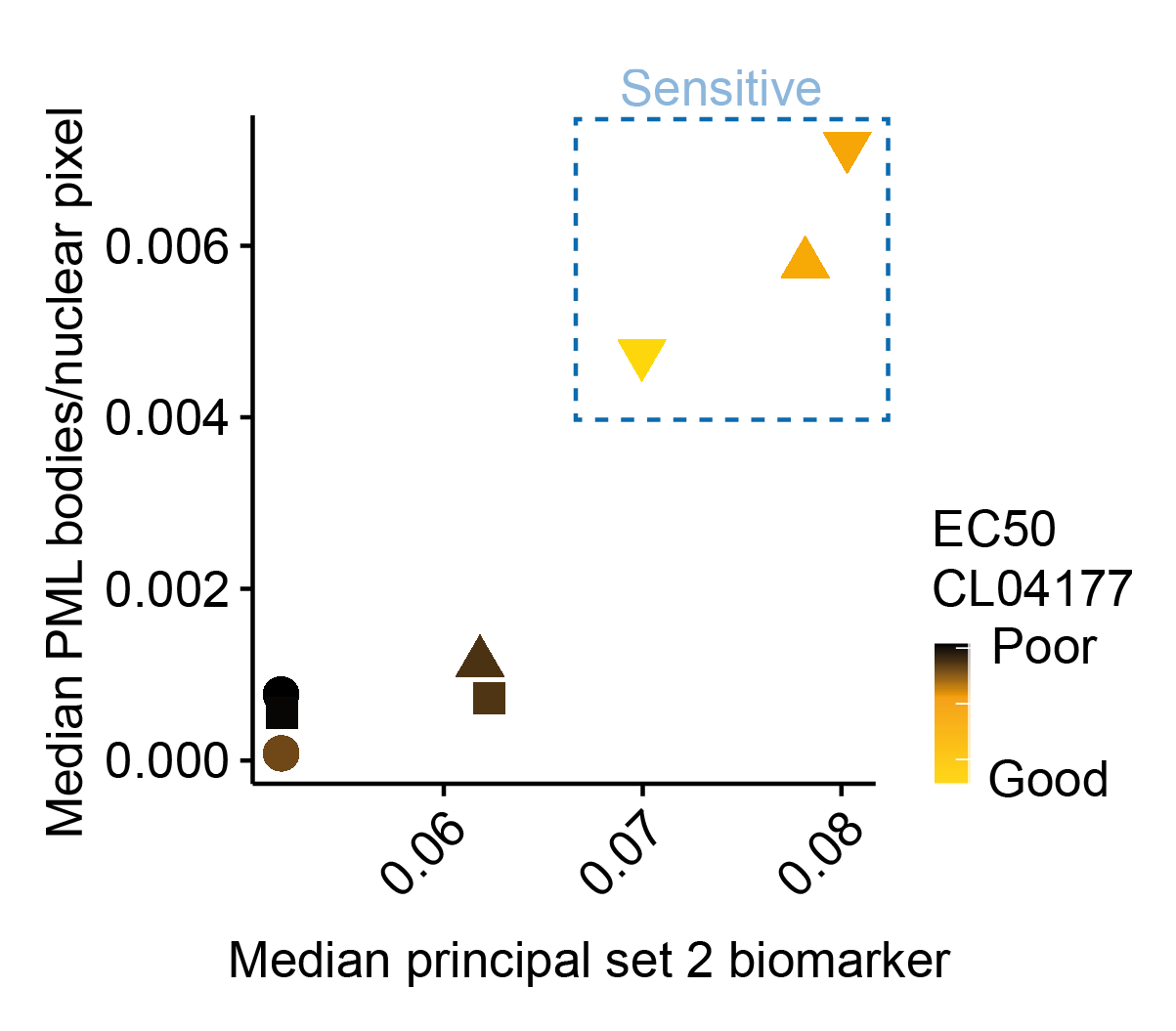
In vitro experiments indicate that, indeed, there is a strong correlation between positivity of PML and sensitivity to CL04177. Further experiments also confirmed that CL04177 could eliminate biomarker-positive 3D patient derived organoid cultures.
Following experiments on stability, pharmacology, kinetics, dynamics and the dose-limiting toxicity, we performed in vivo efficacy experiments. At doses far from the maximally tolerated dose and at dosing schemes that are clinically acceptable, Cleara’s FOXO4 peptides proved to be efficacious in mouse models carrying human cancer cells.
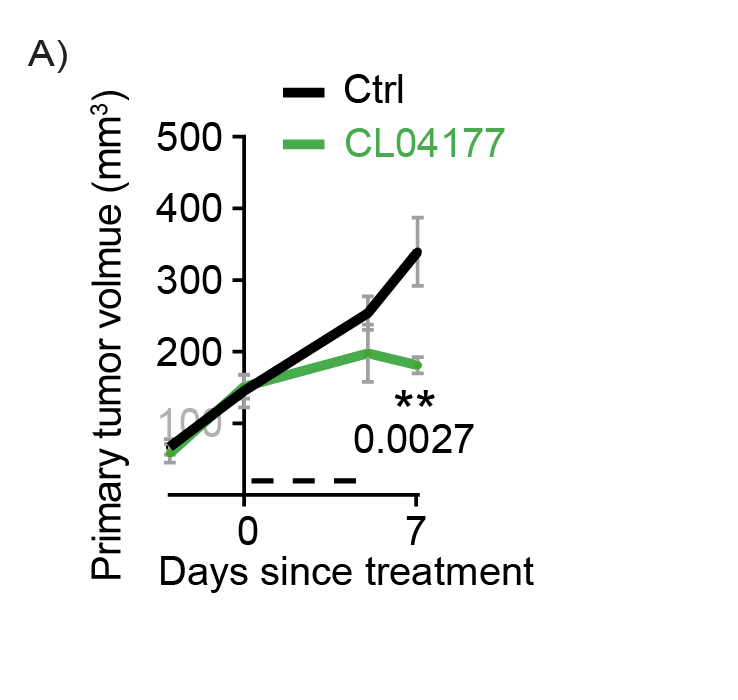
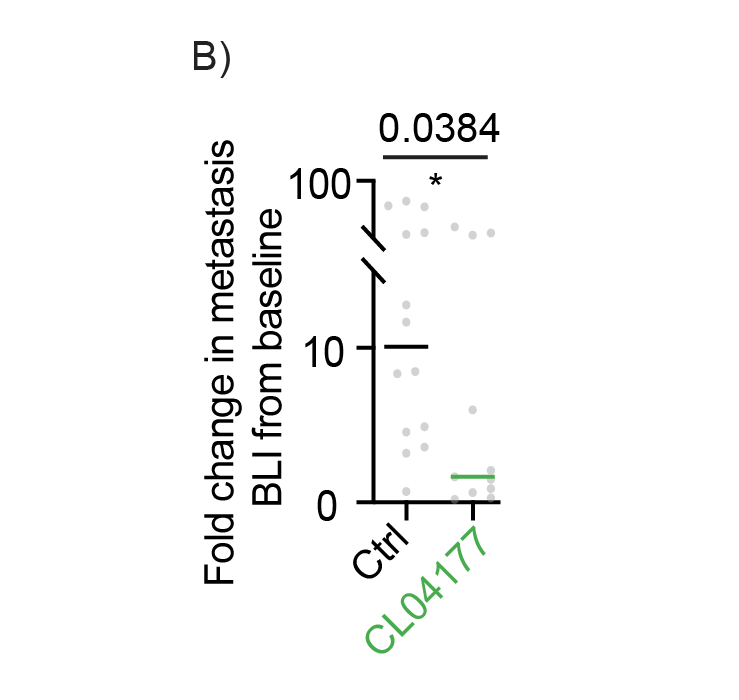
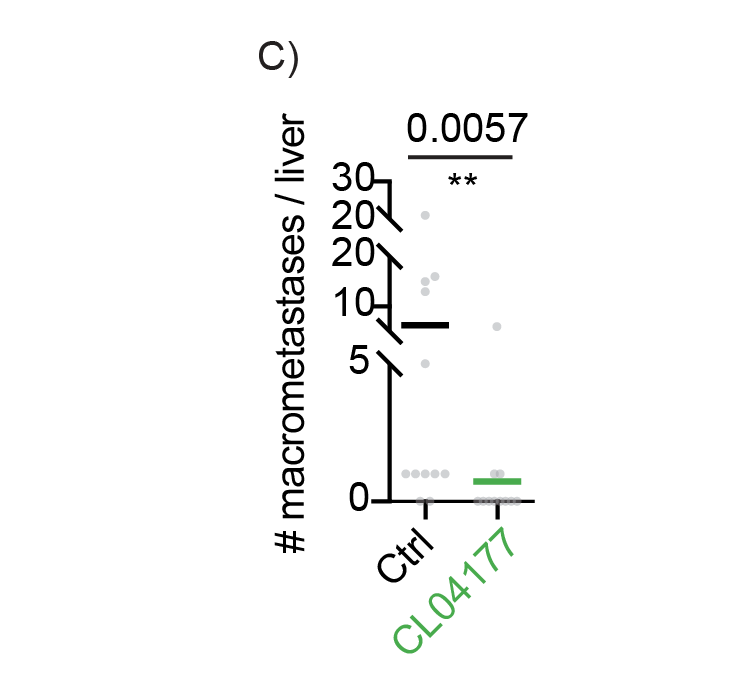
Excitingly, Cleara’s FOXO4-pP53 targeting peptide CL04177 effectively reduces primary tumor growth and metastatic burden of Triple Negative Breast Cancer in vivo. Administered at well below Maximally Tolerated Dose, CL04177 effectively stalls primary tumor growth (A), and reduces metastatic load as determined by bioluminescence 3 weeks after treatment initiation (B), an effect also visible through post mortem quantification of the number of metastases in tissue sections (C).

